TYPES OF HYDROGEN
Hydrogen can be sorted into various groups based on the techniques employed to produce it. These categories reflect the different levels of environmental impact and carbon emissions linked to hydrogen production methods. The trend is towards increasing the share of green and blue hydrogen to diminish the carbon footprint of the hydrogen industry and facilitate the transition to a more sustainable energy system. The classification frequently revolves around the carbon discharges linked to each method. Behold some of the most popular groups
Grey Hydrogen
Grey hydrogen is the product of fossil fuels, predominantly natural gas. It is made via steam methane reforming (SMR) or coal gasification. This technique is the most widespread and cost-effective but spews out substantial quantities of carbon dioxide, contributing to greenhouse gas emissions.
Blue Hydrogen
Blue hydrogen is created using the same methods as grey hydrogen but with an additional step of carbon capture and storage (CCS). The carbon dioxide released during hydrogen production is trapped and stored underground, reducing the carbon discharges related to the process.
Green Hydrogen
Green hydrogen is made using renewable energy sources, such as solar, wind, or hydroelectric power, by means of electrolysis. Renewable electricity is employed to divide water into hydrogen and oxygen, without any carbon emissions. Green hydrogen is regarded as the most eco-friendly and sustainable method of hydrogen production.
Turquoise Hydrogen
Turquoise hydrogen is produced by combining natural gas and renewable energy sources. The natural gas is partially oxidized with oxygen or steam, and the ensuing carbon emissions are captured and stored via CCS. The renewable energy component helps decrease the overall carbon footprint of the production process.
Brown Hydrogen
Brown hydrogen refers to hydrogen made from coal by employing gasification processes. It is an older and less frequent method that isn’t widely used today due to its high carbon emissions and detrimental environmental impact.
Purple Hydrogen
Purple hydrogen is obtained via nuclear energy as the primary power source for electrolysis. Nuclear power supplies the electricity required to divide water into hydrogen and oxygen, resulting in zero carbon emissions during the production process.
What is green Hydrogen?
Green hydrogen is generated by harnessing the power of renewable sources, like solar, wind, or hydroelectricity. This involves the electrification of water, which undergoes a split of its atomic components – oxygen and hydrogen – through an electric current. This current is powered by clean energy sources, ensuring a minute carbon footprint.
The term “green” in green hydrogen depicts its eco-friendly aspect. Unlike traditional hydrogen manufacturing processes, which depend on fossil fuels such as natural gas, green hydrogen production has no greenhouse gas emissions. It is perceived as a sustainable and clean energy carrier that can be used for various purposes, including transportation fuel, industrial processes, and energy storage.
Green hydrogen has the potential to decarbonize sectors that are arduous to electrify directly, such as long-distance transportation and heavy industry. Its adoption can contribute to the reduction of carbon dioxide emissions and facilitate the transition to a sustainable and low-carbon energy system.
What is inside the Green Hydrogen Plant?
These constituents collaborate to facilitate the production, storage, and distribution of green hydrogen, providing a sustainable and low-carbon energy carrier. A verdant hydrogen production facility, utilizing renewable energy sources, typically comprises of the following components:
Renewable Energy Sources
Green hydrogen production plants depend on renewable energy sources such as solar, wind, or hydroelectric power. These sources provide the electricity required for the electrolysis process.
Electrolyzer
The electrolyzer is an indispensable component that executes the electrolysis process. It comprises of an anode and a cathode separated by an electrolyte. When an electric current is applied, water (H2O) is cleaved into hydrogen (H2) at the cathode and oxygen (O2) at the anode.
Power Conversion System
The power conversion system is liable for converting the electrical energy from the renewable energy sources into the appropriate form for the electrolyzer. It may incorporate transformers, inverters, or other power conditioning equipment.
Water Treatment and Purification
Water is a fundamental input for the electrolysis process, and it requires treatment and purification before entering the electrolyzer. This typically entails filtration, demineralization, and removing impurities that could impact the effectiveness and durability of the electrolysis process.
Hydrogen Compression and Storage
After production, the hydrogen gas necessitates compression and storage for future use. Compressors are utilized to boost the pressure of the hydrogen gas, making it suitable for storage and transportation. Storage options may include compressed gas cylinders, subterranean caverns, or other hydrogen storage technologies.
Purification and Conditioning
Depending on the specific requirements of the application, the yielded hydrogen may require further purification and conditioning. This could involve eliminating impurities such as moisture, carbon dioxide, or other trace elements to meet the desired quality standards.
Safety Systems
Safety systems play a pivotal role in hydrogen production plants. These encompass monitoring and control systems to ensure safe operations, leak detection systems, fire suppression systems, and other measures to handle potential hazards associated with hydrogen.
Distribution and Transportation
Green hydrogen produced at the plant may be distributed and transported to various end-use applications. This can involve pipelines, tanker trucks, or other means of transportation depending on the scale and location of the plant.
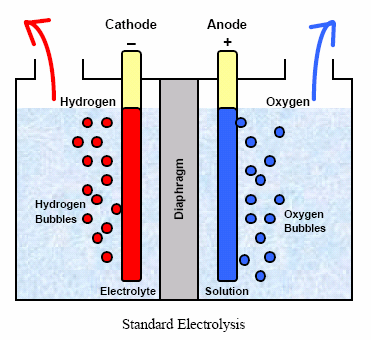
What is Electrolysis
Electrolysis is a chemical process that uses an electric current to drive a non-spontaneous redox reaction. It involves the decomposition of a compound into its constituent elements or ions using an electrolytic cell.
In the process of electrolysis, an electrolyte, a substance that conducts electricity when dissolved or molten, is introduced into the electrolytic cell. This electrolyte could either be an ionic compound or a solution containing ions. Two electrodes, an anode (a positive electrode) and a cathode (a negative electrode), are submerged in the electrolyte.
Once an electric current is activated on the electrodes, positive ions (known as cations) migrate towards the cathode, where reduction takes place, while negative ions (anions) move towards the anode, where oxidation occurs. At the electrodes, ions acquire or lose electrons, resulting in the desired chemical reactions.
when water (H2O) is electrolyzed, hydrogen gas (H2) is produced at the cathode, and oxygen gas (O2) is generated at the anode.
Different Types of Electrolyzers
The hydrogen production process through electrolysis utilizes various types of electrolyzers. It’s noteworthy that these electrolyzers differ in operating temperatures, electrolyte materials, efficiency levels, and application suitability. The type of Electrolyzer employed is determined by several factors, including the scale of production, the desired hydrogen purity, efficiency requirements, and cost considerations. Below are the three most common types of electrolyzers in use:
Proton Exchange Membrane (PEM) Electrolyzer
PEMs are a temperature-savvy option renowned for their rapid response times and high-efficiency levels. They utilize a solid polymer electrolyte membrane composed of a proton-conducting polymer and only require pure water as an electrolyte. PEMs are ideal for small to medium sized applications such as hydrogen refueling stations or on-site hydrogen generation.
Alkaline Water Electrolyzer (AWE)
AWEs are a well-established and mature electrolysis technology. They operate at higher temperatures and utilize an alkaline electrolyte, typically a solution of potassium hydroxide (KOH). AWEs offer high hydrogen purity, and their efficiency can be amplified by the use of waste heat or electricity. They are commonly used for larger-scale applications such as industrial hydrogen production or grid-scale energy storage.
Solid Oxide Electrolyzer Cell (SOEC)
SOECs operate at high temperatures and employ a solid oxide ceramic electrolyte. They have the ability to efficiently utilize a broad range of feedstocks, including steam and carbon dioxide, making them suitable for co-electrolysis or other advanced hydrogen production methods. SOECs are primarily used in large-scale applications that require high efficiency, such as integrated energy systems or industrial processes.
Renewable Energy & Green Hydrogen
Renewable energy and green hydrogen are intimately linked in the realm of hydrogen production. Renewable energy sources, like solar, wind, and hydroelectric power, generate electricity sans any direct emission of greenhouse gases. To power the electrolysis process, which cleaves water into hydrogen and oxygen, green hydrogen production harnesses this clean and sustainable electricity.
The connection between renewable energy and green hydrogen empowers the production of a clean, sustainable, and versatile energy carrier, which can contribute to the transition to a low-carbon economy and help mitigate climate change.
The utilization of InSolare energy for green hydrogen fosters a sustainable energy ecosystem that promotes resilience, reduces reliance on fossil fuels, and mitigates climate change. By combining the benefits of solar power, wind power and green hydrogen, InSolare plays a vital role in helping you with your journey towards a net-zero carbon economy, offering a pathway to a sustainable and greener future.
Here’s the scoop on the connection between renewable energy and green hydrogen:
Electricity Generation
Renewable energy sources generate electricity without relying on fossil fuels, thus cutting down or eliminating carbon emissions. Solar panels transmute sunlight into electricity, wind turbines produce power from wind energy, and hydroelectric plants capitalize on the energy of flowing water. The renewable electricity generated by these sources serves as the primary energy input for green hydrogen production.
Electrolysis
The electrolysis process is the crucial step in green hydrogen production. It necessitates passing an electric current through water (H2O) in an electrolyzer to chop it into hydrogen (H2) and oxygen (O2). This process demands electricity as the energy source, which can be furnished by renewable energy sources.
Carbon-Free Hydrogen Production
By employing renewable electricity for electrolysis, green hydrogen production becomes carbon-free or nearly carbon-free. Unlike hydrogen produced from fossil fuel-based methods, green hydrogen produced from renewable energy does not contribute to greenhouse gas emissions.
Decarbonization Potential
Green hydrogen plays a pivotal role in decarbonizing sectors that are challenging to electrify directly, like heavy industry, transportation, and energy storage. By utilizing renewable energy to produce hydrogen, these sectors can curtail their carbon footprint and shift towards cleaner energy sources.
Energy Storage and Grid Balancing
Green hydrogen can also function as an energy storage medium. Surplus renewable electricity generated during periods of low demand can be utilized to produce hydrogen, which can be stored and later transformed back into electricity when required. This helps balance the intermittent nature of renewable energy sources and guarantees a stable and reliable energy supply.
